Problems and solutions for Atomic Absorption Spectrometry
What is atomic absorption spectrometry principle?
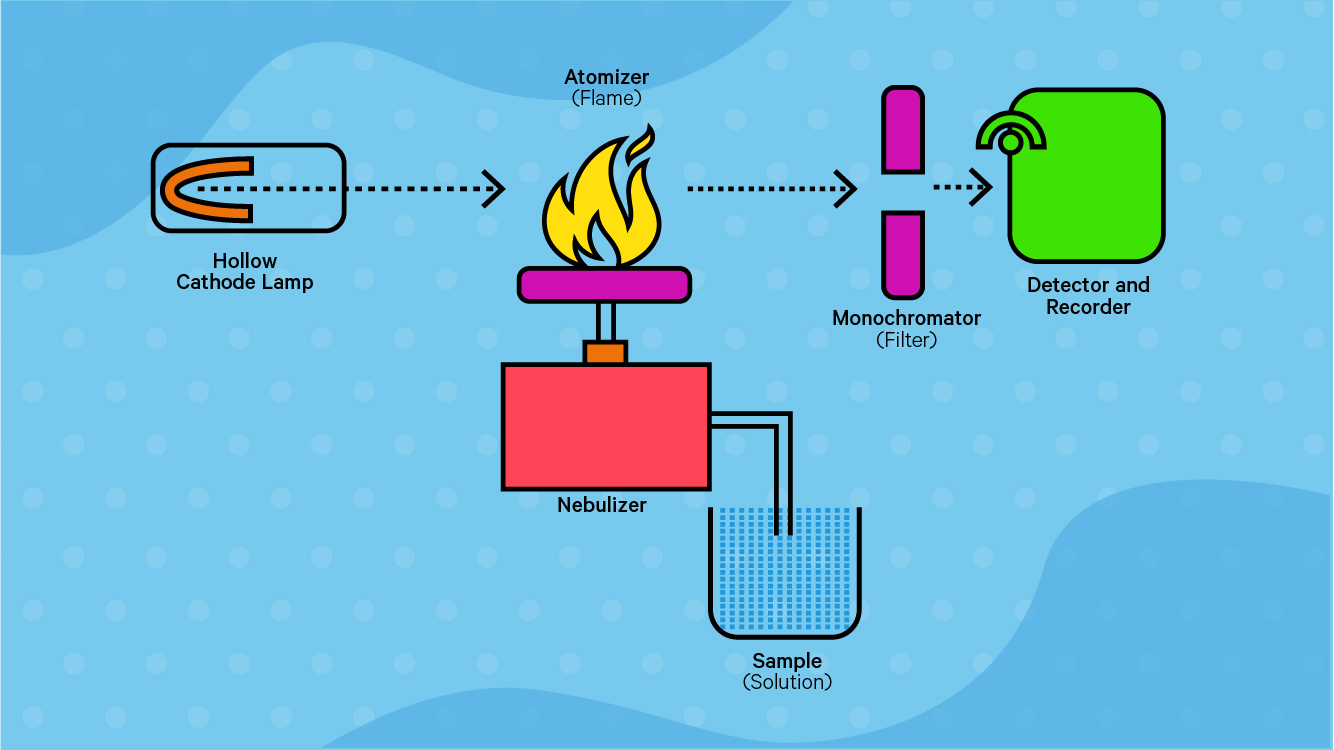
1.The gaseous ground state atoms of the element to be measured in the vapor absorb the characteristic radiation lines of the element to be measured from the light source, with a certain selectivity, and the content of the element to be measured in the sample is obtained by the degree of attenuation of the radiation.
2.When the radiation passes through the atomic vapor, and the frequency of the radiation is equal to the frequency of the energy required for the electrons in the atom to jump from the ground state to a higher energy state, the atoms absorb energy from the incident radiation, producing resonance absorption. Atomic absorption spectra are produced by electrons jumping between the ground state and the first excited state of an atom. The energy level structure of each atom is unique, so atoms selectively absorb radiation frequencies. Therefore, in all cases, an atomic absorption spectrum can be produced that reflects the structural characteristics of that particular atom.
4 classic problems for atomic absorption spectrometry.
1. For a variety of samples have the most adapted to its analytical method, to understand the scope of application of atomic absorption spectrometry, consider its adaptability. As we all know, the graphite furnace atomic absorption of the absolute detection limit value is very high, from this point of view alone, some people wrongly believe that the concentration of high samples with a graphite furnace atomic absorption method is also able to determine, or incorrectly believe that the graphite furnace atomic absorption method to determine the dynamic range is very wide, and has a high degree of accuracy. For example, a manufacturer in the purchase of instruments, that is, the detection limit of good instruments, think that can determine the low concentration of the solution will certainly be able to determine the high concentration of the solution. However, when the instrument is bought back to understand that this judgment is wrong, for the high concentration of the solution must be diluted to the appropriate concentration range to determine. Therefore, for the determination of high-concentration samples, the choice of high-precision measurement methods, such as the choice of spectrophotometry than the choice of atomic absorption method is better. This is because the atomic absorption spectrometry is to measure the absorption of light, and the absorption line and the hollow cathode lamp emission line of the half-width of the ratio of about 10, so can not be like the emission spectrometry, the simultaneous determination of a wide range of concentration of samples.
2. Draw the correct working curve, due to the narrow linear range of the atomic absorption method, so draw the correct working curve is particularly important. In doing the working curve should pay attention to the following points: 1, drawing a working curve at least 5 to 7 points, and each point should be repeated two or more times, until the determination of parallel samples to meet the requirements of the value of the next point of measurement. 2, the standard samples and samples to be measured must be used with the same solvent system. 3, the working curve selected by the concentration range to include the concentration of samples to be measured. Atomic absorption method is more desirable linear range within 0.1 ~ 0.5 of the absorbance, such as the concentration is higher, the standard curve is significantly bent. Therefore, atomic absorption can only determine a narrower concentration range than spectrophotometry. As a remedy, one of the methods is to use a variety of absorption lines with different sensitivities in combination to achieve a wide concentration range. However, this method is not very suitable for alkali metal and alkaline earth metal elements with few absorption lines, and is only barely suitable for elements such as lead, copper, iron, manganese, and platinum. As another remedy is to add a few more points where the working curve starts to bend in order to draw a correct working curve, or use a quadratic equation to draw the working curve.

3. The impact of sample dilution on the results of the analysis of atomic absorption in the field of water quality testing is commonly used in the flame atomic absorption and graphite furnace atomic absorption of two analytical methods. As the sensitivity of the two methods are different, therefore, according to the concentration range of the sample should be selected according to the corresponding analytical method. The working range of different instruments for the same project is different. Before making a sample, you should first be clear about the working range of the instrument you are using. If the concentration range of the sample is not within the working range of your instrument, then you should consider diluting the sample so that the concentration range of the diluted sample is within the working range of the instrument. It is worth noting that the dilution is not too large, and this is especially important when using graphite furnace atomic absorption for detection. This is because the graphite furnace atomic absorption sensitivity is very high, the use of distilled water, deionized water and acid is bound to contain impurities, which will produce measurement errors.
4. The impact of acid on the determination of 1, the impact of the blank value, a few years ago, in the graphite furnace atomic absorption spectrometry to do the standard curve of lead is, suddenly found that the blank value was much higher. At that time, I suspected that the container was contaminated, and then re-wash the container, re-formulated blank solution (with 1% HNO3 deionized water to do the determination of the blank value), the results are still the same, and re-replace the graphite tube also does not work. After repeated tests, it was finally found to be the interference of nitric acid. The nitric acid used at that time was newly opened, and with the last time is not a manufacturer. 2, the impact of sensitivity, due to the production of some manufacturers of nitric acid containing impurities in the amount of higher than the amount labeled on the label, in the graphite furnace atomic absorption spectrometry, so that the sensitivity of the determination of the reduction. If this situation is encountered, re-replace the nitric acid, or in the pre-treatment of water samples, as little as possible, or do not use nitric acid. As the acid produced by different manufacturers, the content of impurities is not the same. Therefore, in the graphite furnace for water analysis, we must pay attention to: with the standard series of acid and water samples added in the acid must be the same manufacturer with the same batch number of acid, only so that the acid on the standard series of determination and determination of water samples on the same level of error control. This is particularly important in graphite furnace atomic absorption analysis.
Precautions
In short, the use of atomic absorption spectrometry for sample analysis, on the one hand, we must have sufficient knowledge of the performance of the instrument; on the other hand, we must continue to sum up experience in practice, improve analytical skills. Only in this way can we obtain satisfactory analysis results.
1.The gaseous ground state atoms of the element to be measured in the vapor absorb the characteristic radiation lines of the element to be measured from the light source, with a certain selectivity, and the content of the element to be measured in the sample is obtained by the degree of attenuation of the radiation.
2.When the radiation passes through the atomic vapor, and the frequency of the radiation is equal to the frequency of the energy required for the electrons in the atom to jump from the ground state to a higher energy state, the atoms absorb energy from the incident radiation, producing resonance absorption. Atomic absorption spectra are produced by electrons jumping between the ground state and the first excited state of an atom. The energy level structure of each atom is unique, so atoms selectively absorb radiation frequencies. Therefore, in all cases, an atomic absorption spectrum can be produced that reflects the structural characteristics of that particular atom.

4 classic problems for atomic absorption spectrometry.
1. For a variety of samples have the most adapted to its analytical method, to understand the scope of application of atomic absorption spectrometry, consider its adaptability. As we all know, the graphite furnace atomic absorption of the absolute detection limit value is very high, from this point of view alone, some people wrongly believe that the concentration of high samples with a graphite furnace atomic absorption method is also able to determine, or incorrectly believe that the graphite furnace atomic absorption method to determine the dynamic range is very wide, and has a high degree of accuracy. For example, a manufacturer in the purchase of instruments, that is, the detection limit of good instruments, think that can determine the low concentration of the solution will certainly be able to determine the high concentration of the solution. However, when the instrument is bought back to understand that this judgment is wrong, for the high concentration of the solution must be diluted to the appropriate concentration range to determine. Therefore, for the determination of high-concentration samples, the choice of high-precision measurement methods, such as the choice of spectrophotometry than the choice of atomic absorption method is better. This is because the atomic absorption spectrometry is to measure the absorption of light, and the absorption line and the hollow cathode lamp emission line of the half-width of the ratio of about 10, so can not be like the emission spectrometry, the simultaneous determination of a wide range of concentration of samples.
2. Draw the correct working curve, due to the narrow linear range of the atomic absorption method, so draw the correct working curve is particularly important. In doing the working curve should pay attention to the following points: 1, drawing a working curve at least 5 to 7 points, and each point should be repeated two or more times, until the determination of parallel samples to meet the requirements of the value of the next point of measurement. 2, the standard samples and samples to be measured must be used with the same solvent system. 3, the working curve selected by the concentration range to include the concentration of samples to be measured. Atomic absorption method is more desirable linear range within 0.1 ~ 0.5 of the absorbance, such as the concentration is higher, the standard curve is significantly bent. Therefore, atomic absorption can only determine a narrower concentration range than spectrophotometry. As a remedy, one of the methods is to use a variety of absorption lines with different sensitivities in combination to achieve a wide concentration range. However, this method is not very suitable for alkali metal and alkaline earth metal elements with few absorption lines, and is only barely suitable for elements such as lead, copper, iron, manganese, and platinum. As another remedy is to add a few more points where the working curve starts to bend in order to draw a correct working curve, or use a quadratic equation to draw the working curve.

3. The impact of sample dilution on the results of the analysis of atomic absorption in the field of water quality testing is commonly used in the flame atomic absorption and graphite furnace atomic absorption of two analytical methods. As the sensitivity of the two methods are different, therefore, according to the concentration range of the sample should be selected according to the corresponding analytical method. The working range of different instruments for the same project is different. Before making a sample, you should first be clear about the working range of the instrument you are using. If the concentration range of the sample is not within the working range of your instrument, then you should consider diluting the sample so that the concentration range of the diluted sample is within the working range of the instrument. It is worth noting that the dilution is not too large, and this is especially important when using graphite furnace atomic absorption for detection. This is because the graphite furnace atomic absorption sensitivity is very high, the use of distilled water, deionized water and acid is bound to contain impurities, which will produce measurement errors.
4. The impact of acid on the determination of 1, the impact of the blank value, a few years ago, in the graphite furnace atomic absorption spectrometry to do the standard curve of lead is, suddenly found that the blank value was much higher. At that time, I suspected that the container was contaminated, and then re-wash the container, re-formulated blank solution (with 1% HNO3 deionized water to do the determination of the blank value), the results are still the same, and re-replace the graphite tube also does not work. After repeated tests, it was finally found to be the interference of nitric acid. The nitric acid used at that time was newly opened, and with the last time is not a manufacturer. 2, the impact of sensitivity, due to the production of some manufacturers of nitric acid containing impurities in the amount of higher than the amount labeled on the label, in the graphite furnace atomic absorption spectrometry, so that the sensitivity of the determination of the reduction. If this situation is encountered, re-replace the nitric acid, or in the pre-treatment of water samples, as little as possible, or do not use nitric acid. As the acid produced by different manufacturers, the content of impurities is not the same. Therefore, in the graphite furnace for water analysis, we must pay attention to: with the standard series of acid and water samples added in the acid must be the same manufacturer with the same batch number of acid, only so that the acid on the standard series of determination and determination of water samples on the same level of error control. This is particularly important in graphite furnace atomic absorption analysis.

Precautions
In short, the use of atomic absorption spectrometry for sample analysis, on the one hand, we must have sufficient knowledge of the performance of the instrument; on the other hand, we must continue to sum up experience in practice, improve analytical skills. Only in this way can we obtain satisfactory analysis results.