Difference between Kjeldahl method and Dumas method
What is Kjeldahl method?
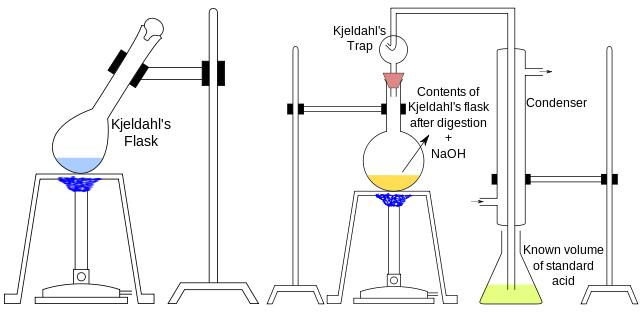
In 1883, the Danish analytical chemist Johan Kjeldahl invented a method for determining the nitrogen content of organic compounds. He will be a certain weight of the specimen and sulfuric acid, so that all the nitrogen in the specimen is converted to ammonium sulfate, and then add alkali to the ammonium sulfate solution, and then the resulting ammonia distilled to a certain volume of standard acid solution, and then titrate the excess acid, you can measure the nitrogen content of the specimen. Later people called this method for Kjeldahl method; Kjeldahl method used in the instrument is a pear-shaped long-necked flask, the capacity is usually about 300 ml, micro-analysis can be as small as 10 ml, later people called this flask for Kjeldahl flask.
What is Dumas method?
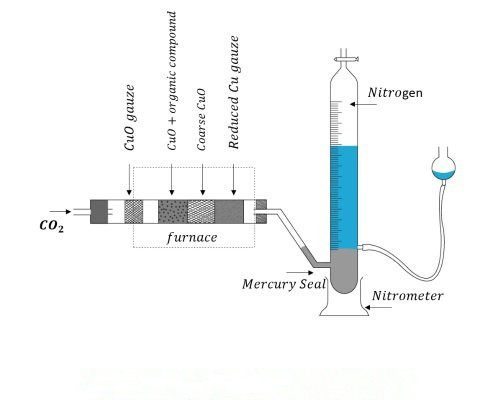
In 1831, the French chemist Jean Baptiste Dumas pioneered a practical method of nitrogen determination. This method is stored in the front of the combustion tube with lead carbonate, in the sample decomposition, heating lead carbonate, so that the decomposition of carbon dioxide completely excluded from the combustion tube of the air sample and CuO combustion, the gas generated by the PbCO decomposition of CO air flow driven to the KOH solution inside the mercury tank on the stand in the collection of gas cylinder. During combustion, some of the nitrogen is occasionally converted to oxides of nitrogen, which are reduced after passing through red-hot copper powder, so that all the nitrogen in the organic matter is reduced to N .
Kjedahl vs Dumas
The principle of the Kjeldahl nitrogen analyzer is the Kjeldahl method Proteins are organic compounds containing nitrogen.
Foodstuffs are digested with sulfuric acid and catalyst together with heating to decompose proteins, and the decomposed ammonia combines with sulfuric acid to produce ammonium sulfate. Alkaline distillation then frees the ammonia, which is absorbed with boric acid and then titrated with a standard solution of sulfuric or hydrochloric acid, and the protein content is multiplied by a conversion factor based on the consumption of acid. Nitrogen content x 6.25 = protein content. The amine root in the organic matter is nitrated to produce (NH4)2SO4 under the action of strong heat and CuSO4 and concentrated H2SO4.1. Kjeldahl nitrogen fixation method reaction formula: 2NH2+H2SO4+2H=(NH4)2SO4 (in which CuSO4 is used as a catalyst).2. Acting with alkali in Kjeldahl nitrogen fixation apparatus, NH3 is released by distillation , and collected in the H3BO3 solution reaction formula is: (NH4)2SO4 + 2NaOH = 2NH3 + 2H2O + Na2SO42NH3 + 4H3BO3 = (NH4)2B4O7 + 5H2O3, titration with a known concentration of H2SO4 (or HCI) standard solution, according to the amount of HCI consumption of nitrogen content, and then multiplied by the corresponding conversion factor, both the content of the protein reaction formula: ( NH4)2B4O7+H2SO4+5H2O=(NH4)2SO4+4H3BO3(NH4)2B4O7+2HCl+5H2O=2NH4Cl+4H3BO3.
The principle of Dumas Nitrogen Determination is the Dumas combustion method
The method of Dumas combustion is to encapsulate a certain amount of sample in an aluminum boat, and then burn it in a high temperature furnace; in the environment of catalyst and oxygen, the gases generated by the combustion of the sample are: CO2, H2O, and NOx; these gases then pass through the reduction furnace, NOx is reduced to N2, and CO2 and H2O are separated; finally, elemental nitrogen is measured by the Thermal Conductivity Detector (TCD) to determine the content. The entire measurement process is completed in less than 3 minutes.
In 1883, the Danish analytical chemist Johan Kjeldahl invented a method for determining the nitrogen content of organic compounds. He will be a certain weight of the specimen and sulfuric acid, so that all the nitrogen in the specimen is converted to ammonium sulfate, and then add alkali to the ammonium sulfate solution, and then the resulting ammonia distilled to a certain volume of standard acid solution, and then titrate the excess acid, you can measure the nitrogen content of the specimen. Later people called this method for Kjeldahl method; Kjeldahl method used in the instrument is a pear-shaped long-necked flask, the capacity is usually about 300 ml, micro-analysis can be as small as 10 ml, later people called this flask for Kjeldahl flask.

What is Dumas method?
In 1831, the French chemist Jean Baptiste Dumas pioneered a practical method of nitrogen determination. This method is stored in the front of the combustion tube with lead carbonate, in the sample decomposition, heating lead carbonate, so that the decomposition of carbon dioxide completely excluded from the combustion tube of the air sample and CuO combustion, the gas generated by the PbCO decomposition of CO air flow driven to the KOH solution inside the mercury tank on the stand in the collection of gas cylinder. During combustion, some of the nitrogen is occasionally converted to oxides of nitrogen, which are reduced after passing through red-hot copper powder, so that all the nitrogen in the organic matter is reduced to N .

Kjedahl vs Dumas
The principle of the Kjeldahl nitrogen analyzer is the Kjeldahl method Proteins are organic compounds containing nitrogen.
Foodstuffs are digested with sulfuric acid and catalyst together with heating to decompose proteins, and the decomposed ammonia combines with sulfuric acid to produce ammonium sulfate. Alkaline distillation then frees the ammonia, which is absorbed with boric acid and then titrated with a standard solution of sulfuric or hydrochloric acid, and the protein content is multiplied by a conversion factor based on the consumption of acid. Nitrogen content x 6.25 = protein content. The amine root in the organic matter is nitrated to produce (NH4)2SO4 under the action of strong heat and CuSO4 and concentrated H2SO4.1. Kjeldahl nitrogen fixation method reaction formula: 2NH2+H2SO4+2H=(NH4)2SO4 (in which CuSO4 is used as a catalyst).2. Acting with alkali in Kjeldahl nitrogen fixation apparatus, NH3 is released by distillation , and collected in the H3BO3 solution reaction formula is: (NH4)2SO4 + 2NaOH = 2NH3 + 2H2O + Na2SO42NH3 + 4H3BO3 = (NH4)2B4O7 + 5H2O3, titration with a known concentration of H2SO4 (or HCI) standard solution, according to the amount of HCI consumption of nitrogen content, and then multiplied by the corresponding conversion factor, both the content of the protein reaction formula: ( NH4)2B4O7+H2SO4+5H2O=(NH4)2SO4+4H3BO3(NH4)2B4O7+2HCl+5H2O=2NH4Cl+4H3BO3.
The principle of Dumas Nitrogen Determination is the Dumas combustion method
The method of Dumas combustion is to encapsulate a certain amount of sample in an aluminum boat, and then burn it in a high temperature furnace; in the environment of catalyst and oxygen, the gases generated by the combustion of the sample are: CO2, H2O, and NOx; these gases then pass through the reduction furnace, NOx is reduced to N2, and CO2 and H2O are separated; finally, elemental nitrogen is measured by the Thermal Conductivity Detector (TCD) to determine the content. The entire measurement process is completed in less than 3 minutes.