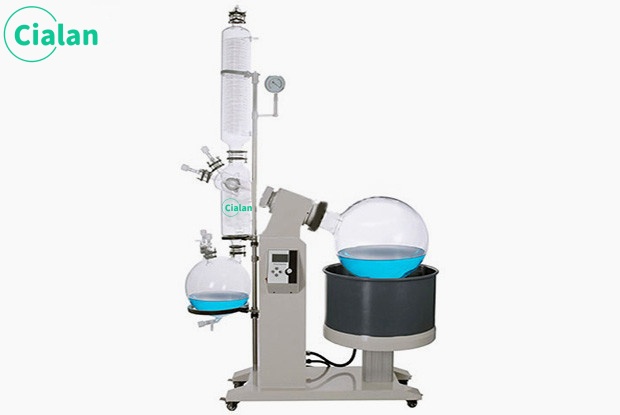
What is fractional distillation and how does it work?
Fractional distillation is a method of separating a mixture of several volatile substances with different boiling points;
A mixture is heated and separated into a relatively pure single substance by cooling for the different boiling points of the components of the mixture. There is no new material produced in the process, but only the original material is separated, which is a physical change.
Fractional distillation is actually multiple distillation, which is more suitable for the separation and purification of liquid organic mixtures with little difference in boiling points. For example, the fractional distillation of coal tar; the fractional distillation of petroleum. When the boiling points of substances are very close to each other, about 20 degrees difference, then simple distillation cannot be used and fractional distillation can be used instead. The small column of a fractionation column provides a large surface area for vapor condensation.
A mixture is heated and separated into a relatively pure single substance by cooling for the different boiling points of the components of the mixture. There is no new material produced in the process, but only the original material is separated, which is a physical change.
Fractional distillation is actually multiple distillation, which is more suitable for the separation and purification of liquid organic mixtures with little difference in boiling points. For example, the fractional distillation of coal tar; the fractional distillation of petroleum. When the boiling points of substances are very close to each other, about 20 degrees difference, then simple distillation cannot be used and fractional distillation can be used instead. The small column of a fractionation column provides a large surface area for vapor condensation.