ATR FTIR Spectrometer FAQ Guide : A Must-Read For Laboratory Equipment Buyers
Are you looking to add a high performance FTIR Spectroscopy to your laboratory or colleague? Consider Fourier Transform Infrared Spectrometer machine has important applications in many fields, and it has a number of unique advantages and performance to meet the needs of different areas of testing, choosing the right one can be challenging . That's why we've created this comprehensive FAQ guide to help you make an informed decision. We'll address all your questions about conception, working principle, operation, samples, difference, advantages, and the limitations.

What Is FTIR Spectroscopy?
FTIR Spectroscopy, also referred to as Fourier Transform Infrared Spectroscopy or FTIR Analysis, is a method of analysis employed for the identification of organic, polymeric, and inorganic substances. FTIR instruments capture frequencies all at once, leading to quicker data collection, better signal to noise ratios, and more precise wavenumber readings.

How Much Does An FTIR Spectroscopy Instrument Cost?
FTIR spectrometer price typically range from $15,000 to $145,000, depending on its resolution, sensitivity, automation or software, brand, model. For seeking a cost-effective option, you can ask your preferred FTIR manufacturer and supplier to provide a model and quotation of FTIR machine according to your requirements.
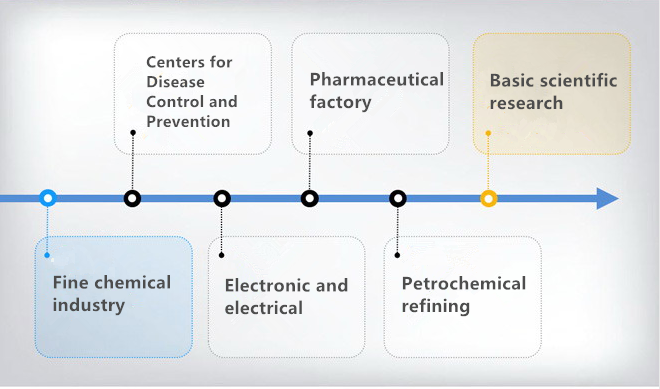
What Is FTIR Spectroscopy Used For?
These applications of FTIR spectrometer demonstrate its importance as a versatile and efficient analytical tool that provides fast, accurate chemical information to help industries improve product quality, optimize production processes, and ensure environmental safety.
Pharmaceutical industry:
For drug development, quality control and biomedical research. It can analyze the composition, structure and purity of drugs.
Drug composition analysis: FTIR machine is used to confirm the chemical structure of APIs and to ensure the purity and quality of chemicals used in the production process.
Quality control: monitoring the quality of drugs in the production process to ensure that the final product meets the preset chemical and physical standards.
Formulation development: Analyzing the interaction of ingredients in drug formulations to optimize drug formulations.
Minerals:
For mineral analysis to help identify mineral types and structures.
Petroleum:
Crude oil and fuel analysis: Analyzing the chemical composition of crude oil and petrochemical products to optimize processing. Analysis of petroleum products to detect organic functional groups and chemical composition.
Coal:
For coal analysis to study the content and structure of organic matter.
Environmental protection:
Pollutant Detection: Monitoring and analyzing pollutants in air, water and soil samples, such as industrial emissions and detection of hazardous chemicals.
For environmental monitoring to analyze pollutants in the atmosphere, water and soil.
Gem identification:
Detect the lattice structure and composition of gemstones and identify the authenticity of gemstones.
Criminal investigation identification:
Analyze the composition of unknown substances to help solve cases.
Conservation and Archaeology:
Material analysis of artifacts: Determine the material composition of ancient objects such as paintings, sculptures, and other artifacts to guide conservation and restoration work.
Food industry:
Ingredient verification: Determining the composition of foodstuffs, such as fats, proteins and other nutrients.
Adulteration Detection: Detects whether food products contain illegally added substances or are adulterated with low-cost substitutes.
Chemical industry:
Chemical analysis: to confirm the structure and purity of products of chemical reactions and raw materials.
Process control: Monitor intermediates and products in chemical processes to optimize productivity and product quality.
Polymer and plastic industry: material performance evaluation, analyze the chemical structure of plastics and polymers, predict and test their physical properties.
Research and development of new materials: develop new plastic formulations and composite materials to meet specific industrial needs.

What Is The Working Principle Of FTIR Spectroscopy?
FTIR spectrometry is an infrared spectroscopy analysis instrument based on the principle of interferometer and Fourier transform.
Interferometer part: FTIR testing generates two coherent infrared light beams with a small optical path difference through the Michelson interferometer. After passing through the sample, these two beams of light interfere with each other to form an interference pattern containing sample information.
Fourier transform part: The interference pattern is input into the computer, and the time domain function (interference pattern) is converted into a frequency domain function (infrared spectrum) through the Fourier transform algorithm. In this way, the infrared spectrum information of the sample can be obtained.

What Is The Structure Of FTIR Spectroscopy?
The basic structure of FTIR spectroscopy instrumenation consists of infrared light source, beam splitter, interferometer, cuvette, detector, computer data processing system and recording system.
1. Infrared light source: provide infrared radiation, commonly used tungsten lamps, tungsten iodine lamps, silicon carbon rods, high-pressure mercury lamps and thorium oxide lamps.
2. Beam splitter: Divide the incident beam into two parts, reflection and transmission, and then make it compound.
3. Interferometer: The interferogram of incident light is obtained using a Michelson interferometer.
4. Sample cell: Place the sample to be tested to ensure that the sample is under the irradiation of infrared light.
5. Detector: Convert the optical signal into an electrical signal. Commonly used detectors are titanium sulfate triglyceride (TGS), barium strontium niobate, mercury cadmium telluride, indium antimonide and so on.
6. Computerized data processing system: control the operation of the instrument, collect and process data.
7. Recording system: record and display the spectral map, in order to analyze and explain.

What Does FTIR Spectroscopy Use To Measure?
FTIR analysis, a widely used technique nowadays, is favored for its sensitivity, versatility, precision, and resilience. It is capable of analyzing solids, liquids, and gases, making it a highly utilized analytical method in the field of science.(Such as Acetone, Methanol, Nitromethane, Benzene, Cyclohexane, Ether, Carbonyl, Polystyrene, Acetic acid, Aspirin, Polypropylene, Polyethylene, Carboxylic acid, Ethyl acetate, Hexane, Amide, Benzoic acid, Methane, Cinnamaldehyde, Ester, Pvc, PP, Silicate FTIR)

What Cannot Be Detected By FTIR Spectroscopy ?
Certain Low Concentration Substances: ATR FTIR spectrometer test has limited detection sensitivity and may have difficulty detecting substances at very low concentrations.
Substances with similar structure: For substances with very similar structure, benchtop FTIR may be difficult to distinguish. In such cases, it may not be possible to accurately determine exactly which substance is involved by relying solely on FTIR testing.
Specific components in partially complex mixtures: In complex mixtures, certain substances may be interfered with by the signals of other components, making them difficult to detect.
Colorless, odorless substances with weak infrared absorption: Some colorless, odorless substances with weak absorption in the infrared spectral region may be difficult to detect by FTIR machine, such as simple cations and anions.
Certain Special Forms of Substances: FTIR spectrometer may be limited in the detection of certain special forms of substances, such as nanoscale particles or ultra-thin films. Metals and some minerals don't absorb light in the infrared range, so they can't be detected by FTIR analyzer. Diatomic or noble gases can not be analysis too.
Substances under extreme conditions such as high temperatures and pressures: Under extreme conditions such as high temperatures and pressures, the structure and properties of a substance may change, thus affecting the FTIR detection results.
Substances with strong fluorescence or phosphorescence: Fluorescence and phosphorescence produce strong light signals, which may mask the absorption signals of infrared light, thus affecting the FTIR detection results.

Can FTIR Spectroscopy Be Used For Qualitative Or Quantitative Analysis?
ATR FTIR spectrometer is well-known for its ability to do qualitative analysis, but it can also be used to do quantitative analysis. Researchers can create calibration curves by analyzing the connection between the strength of infrared absorption bands and the amount of a substance present, allowing for the accurate quantification of target compounds.

What Precautions Should Be Taken When Using FTIR Spectroscopy?
Before Use:
Proper Sample Preparation: Ensure that no impurities are introduced or contamination of the sample is caused during sample preparation.
During use:
Operator Safety: ATR FTIR instruments typically use an infrared light source, and operators should avoid direct exposure to infrared light to avoid eye and skin damage. Appropriate protective eyewear and gloves should be worn when operating the instrument. Not using explosive or flammable samples, not placing anything on top of the electronics cover
Instrument Safety: Ensure that the power connection to the FTIR spectrophotometer is stable to avoid electrical accidents. Be careful when moving or handling the instrument to avoid collision and damage.
Instrument parameter settings: Select these parameters according to the different analytical purposes and sample characteristics.
Baseline correction: In FTIR analyzer, baseline drift may affect the accurate measurement of peak positions and intensities. Choose an appropriate baseline correction method to ensure that the corrected spectrum can accurately reflect the characteristics of the sample.
Peak identification and attribution: Accurate identification and attribution of absorption peaks in an FTIR spectrum is critical to analyzing a sample.
Use of data analysis software: Choosing the appropriate data analysis software can improve the efficiency and accuracy of data processing.
After use:
Instrument maintenance and calibration: Maintain and calibrate the FTIR spectrometry regularly to ensure stable performance and accurate measurement results.
Application Specific Precautions
Cultural relics protection field: in the protection of cultural relics, FTIR technology can be used to analyze the material composition of cultural relics, degradation and so on. However, attention needs to be paid to the protection of cultural relics when using it to avoid damage to the relics.
Medical field: In the medical field, it can be used for disease diagnosis, drug analysis and so on. However, the complexity and diversity of the samples, as well as medical ethics and other issues need to be considered when using it.
Environmental monitoring field: In environmental monitoring, it can be used to detect atmospheric pollutants, water quality and so on. However, attention should be paid to the influence of environmental factors on the measurement results, such as temperature and humidity.
Material Science: In material science, it can be used to analyze the structure and composition of materials. However, when using it, it is necessary to pay attention to the influence of material properties and preparation methods on the measurement results.
Automotive engineering: In automotive engineering, it can be used to analyze the composition of engine exhaust gases. However, attention needs to be paid to the measurement conditions and data processing methods when using it to ensure the accuracy of the results.

How Does FTIR Spectrometer Work/Operate?
Let's take Cialan's FTIR-850 as an example.
1. Press the power switch to turn on the power supply, the instrument needs a self-test process after powering up, about 10 seconds. Wait for at least 15 minutes after the instrument is powered up, and wait for the light source energy output to be fully stabilized before measurement.
2. Turn on the computer and run the operation software. Check whether the communication between the computer and the instrument is normal.
3. Click the “acquisition” menu under the “acquisition settings”, select “Diagnostics” to observe whether the normal, the normal selection of the “optical table “, in the “optical table pane” to observe the energy value, to ensure that the energy value in the acceptable range, such as not in this range, it is necessary to click the “Diagnostics” in the “Collimation If not, you need to click the “Collimation” button in “Diagnostics” to perform the collimation operation.
4. Potassium bromide tablet method: take about 0.3mg of the test article, put it in the agate mortar, add dry potassium bromide, fully grind and mix it, move it to the press mold to make the distribution uniform, place the press mold horizontally on the seat of the tablet press, pressurize it to 10t/cm2, keep it for 3 minutes, take out the test tablet, and check it visually that it should be uniform, with a smooth surface, and with good transmittance of light.
5. Collect background: Click “Collect” menu under the “Collect Background”, appear to collect background prompt box, take out the sample from the sample chamber, insert the blank into it (or nothing), click “OK! Click “OK” to start collecting background.
6. Collect samples: Click the “Collection” menu under the “Collect Samples”, the collection of samples prompt box appears, take the blank out of the sample chamber, insert the sample. Click “OK” to start collecting samples. After the collection, the computer will automatically calculate and display the infrared spectrum of the sample on the spectrum window.
7. Data processing: After the acquisition is completed, the user can carry out the corresponding data processing on the spectrogram, such as labeling peaks, multiplying the spectrogram, smoothing, library search, etc.
8. Press the switch of the main power of the instrument to turn off the power of the main unit.
9. Remove the samples from the sample compartment, make sure the sample compartment is clean, and place bagged desiccant into the sample compartment.
10. Clean the spectrometer and molds; turn off the computer.
11. Make a record of instrument use.

How To Evaluate FTIR Results?
To analyze the results of FTIR spectra, the following steps can be followed:
1. Observe the overall characteristics of the spectrogram:
Examine the horizontal and vertical axes of the spectrogram to understand the relationship between wave number or wavelength and absorbance. Wave number is usually measured in centimeters-1 and absorbance is the degree to which light is absorbed by a substance.
Identify absorption peaks in a spectrogram that indicate the frequency at which a substance absorbs infrared light. The location and intensity of the absorption peaks can provide information about the functional groups of the substance.
2. Identify characteristic absorption peaks:
Each functional group has specific infrared absorption peaks, for example:
Hydroxyl (-OH) usually has an absorption peak between 3200-3600 cm-1.
Carbonyl (C=O) usually has an absorption peak between 1600-1800 cm-1.
Methyl (-CH3) usually has an absorption peak between 2800-3000 cm-1.
Identify functional groups that may be present in the sample by comparing known standard spectra or using specialized software.
3. Analyze the details of the spectrogram:
Check whether the baseline of the spectrogram is smooth, if there is drift or trailing, appropriate processing (e.g. automatic baseline correction and smoothing) is required.
Pay attention to peak shapes and symmetries in the spectrogram; these features can provide information about the structure of the molecule.
If required, the spectrogram can be further analyzed quantitatively, e.g., by calculating the peak areas to determine the content of the substance.
4. Combination with other analytical techniques:
FTIR spectrograms are often used in combination with other analytical techniques to obtain more comprehensive information about a substance.

How To Read FTIR Spectra?
1. Identification of functional groups: Based on the position and shape of the absorption peaks, the presence of functional groups or chemical bonds in the sample can be initially identified.
2. Comparison of samples: The spectra of the samples to be measured are compared with known standards or literature data to confirm the chemical composition and structure of the samples.
3. Quantitative analysis: Through the comparison of peak intensity and integral area, quantitative analysis can be carried out, such as determining the content of a functional group or compound in the sample.
4. Structural inference: According to the position and intensity of the absorption peaks, the molecular structure, conformation and chemical environment of the sample can be inferred.

How Do You Identify FTIR Peaks?
1. Position of peaks (wave number): The position at which each peak appears (expressed in wave number, i.e., cycles per centimeter, cm -1,) tells us which type of chemical bond or functional group is absorbing. The wave number is an indicator of the energy state of the chemical bond, and different types of chemical bonds absorb infrared light in different wave number regions.
2. The shape and width of the peaks:
Sharp peaks: usually indicate the presence of purity in the sample
Sharp peaks: usually indicate the presence of compounds of higher purity in the sample, because the absorption peaks of specific chemical bonds of a single compound are usually sharp and well-defined. 4.
Wide peaks: may indicate that the compounds contained in the sample are more complex or mixtures, as multiple compounds are present.
Wide peaks: may indicate that the sample contains complex compounds or mixtures of compounds, as the overlapping absorption of multiple compounds can lead to broad peaks.
3. Intensity of peaks:
Strong peaks: If a peak is very strong, this usually indicates that the
If a peak is strong, this usually indicates a high concentration of the chemical bond or functional group.
Weak peaks: Conversely, a weak peak indicates a lower concentration of the chemical bond or functional group.
4. Number of peaks: The number of peaks can reflect the complexity of the sample. Multiple peaks may indicate that the sample contains multiple bonds or functional groups.

How To Calculate Frequency From FTIR Graph?
To calculate the frequency, start by acquiring the FTIR spectrum of your sample. Make sure the representation is precise by carrying out any required baseline adjustments. Recognize the significant peaks that correspond to functional groups or chemical bonds present in the sample. Begin by concentrating on the high-frequency portion of the spectrum, as this is important for identifying functional groups.
Once you have found the highest points, establish the area to focus on by observing the span of wavenumbers. This stage is crucial for separating particular vibrations for additional examination or evaluation. Utilize numerical integration methods to compute the extent beneath each line within the specified range. Adding up these extents will yield the overall area beneath the line, which corresponds to the sample's vibrational energy.
Keep in mind that the maximum resolution of your FTIR spectrometer, which is influenced by the movable mirror's range of motion, can impact the accuracy of your frequency measurements. Consider this variable to optimize resolution in

What Are The Advantages Of FTIR Spectroscopy?
High sensitivity: FTIR spectrometer boasts remarkable sensitivity, enabling the identification of minute quantities of substances within a sample.
Enhanced Frequency Accuracy: This technique offers superior frequency resolution and reliability, leading to more precise results.
Wide applications: FTIR spectroscopy is adaptable to a diverse range of samples, including solids, liquids, film and gases.
Non-destructive of Samples: As a that samples remain intact and unaltered throughout the analysis process, ideal for analyzing delicate.
Analysis times are remarkably short, typically spanning from a few seconds to a few minutes, facilitating rapid decision-making.
Quantitative analysis: FTIR spectroscopy allows for the precise quantification of compound concentrations within a sample, providing actionable insights.
Scan faster: yielding a significantly improved signal-to-noise ratio and higher-quality spectral data.
Spectral Storage and Processing: FTIR spectroscopy facilitates the storage and processing of spectral data, streamlining data management and analysis.

What Are The Disadvantages/Limitations Of FTIR Analysis?
ATR FTIR spectrometer is limited to identifying functional groups rather than individual molecules in a sample, making it unsuitable for determining the complete chemical structure of a compound.
Complex samples, such as mixtures or those with overlapping spectra, pose challenges in accurately identifying components.
Additionally, the sensitivity of FTIR to water means that samples must be thoroughly dried before analysis.
The technique also requires the preparation of a thin sample film, which can be time-consuming and necessitates expertise. Spectral interference from other molecules or impurities can lead to inaccurate measurements, requiring the material being tested to be transparent in the relevant spectral region.
The molecule under analysis must also exhibit activity in the infrared range.

What Is The Difference Between FTIR And IR?
Principle:
IR: The structure and composition of a substance is analyzed by using its absorption properties of infrared light at different wavelengths. By measuring the intensity of the sample's absorption of infrared light at different wavelengths, an infrared absorption spectrum can be obtained.
FTIR: Based on the Fourier Transform principle, the interferogram is converted into an infrared spectrum. an FTIR instrument usually consists of an infrared light source, an interferometer, a sample chamber, and a detector. an FTIR has higher resolution and faster scanning speeds, and can measure the entire infrared spectral range at the same time.
Resolution:
FTIR has a higher resolution: FTIR can achieve very high resolutions, typically 0.1 - 0.5 cm-¹ or even higher.
This enables FTIR to resolve very fine spectral features, which is useful for analyzing complex molecular structures and mixtures.
The resolution of IR is relatively low: conventional IR instruments typically have a resolution of a few centimeters-¹ or so. While this may be sufficient for simple analytical tasks, FTIR is more advantageous when high resolution is required.
Scanning speed:
FTIR scans quickly: FTIR utilizes the Fourier Transform technique to scan the entire infrared spectral range in a very short time. This makes FTIR ideal for rapid analysis and real-time monitoring.
Slow IR scanning speed: Conventional IR instruments usually scan for different wavelengths of infrared light point by point, which makes the scanning speed slow. This limits the use of IR in applications that require rapid analysis.
Areas of application:
Common application areas: IR and FTIR have a wide range of applications in several fields, including chemistry, materials science, biomedical, and environmental science.
Unique applications of Benchtop FTIR in certain fields: FTIR machine has unique applications in specific fields due to its higher resolution and faster scanning speed. In cell biology, FTIR can be used to analyze the chemical composition and structure of cells, to detect cellular lesions, and so on.
What Is The Difference Between NIR And FTIR?
Principle
NIR: Near-infrared spectroscopy is the multiplicative and combinatorial frequency absorption spectra of molecular vibration, mainly reflecting the multiplicative and combinatorial frequency vibration information of hydrogen-containing groups (e.g. C-H, O-H, N-H, etc.). Near-infrared light has low energy, and the interaction with molecules is mainly generated by the change of dipole moment caused by molecular vibration. Near-infrared spectroscopy is based on the absorption and scattering of near-infrared light by the material under test, and the spectral response of the sample in the near-infrared region is measured to obtain information on the chemical composition and physical properties of the sample.
FTIR: Fourier Transform Infrared Spectroscopy is based on the absorption properties of molecules to infrared light. When a sample is irradiated with infrared light, the chemical bonds in the molecules will absorb infrared light of a specific wavelength, causing a jump in the vibrational energy levels of the molecules. By measuring the degree of absorption of the sample to different wavelengths of infrared light, the infrared absorption spectrum of the sample can be obtained.FTIR technology utilizes the Fourier transform to convert the interferogram into a spectrogram, which has the advantages of high resolution, high sensitivity and fast measurement.
Technical characteristics
NIR:
Rapid analysis: measurements are fast and a measurement can usually be made in a few seconds, making it suitable for on-line testing and real-time monitoring.
Non-destructive: non-destructive to the sample, allowing non-destructive testing, suitable for precious samples or samples that need to be tested repeatedly.
Simple operation: the instrument is relatively simple to operate, without the need for complex sample preparation process, usually only need to carry out simple processing or direct measurement of the sample.
Multi-parameter simultaneous determination: Multiple chemical compositions can be determined simultaneously, providing rich sample information.
FTIR:
High resolution: with high resolution, it can distinguish subtle spectral differences, which is advantageous for the analysis of complex samples.
Wide applicability: Can analyze various types of samples, including solids, liquids and gases, suitable for applications in different fields.
Accurate structural analysis: able to provide molecular structure information, which plays an important role in the identification and structure analysis of unknown substances.
Strong quantitative analysis capability: accurate quantitative analysis is possible through the establishment of standard curves.

What Is The Difference Between FTIR Spectroscopy And Raman Spectroscopy?
Spectroscopic Principle Aspects
FTIR Fourier Transform Infrared Spectrometer: The vibrational information of molecules is obtained by measuring their absorption of infrared light. Different chemical bonds have specific vibrational frequencies, so that functional groups in a molecule can be identified by infrared spectroscopy.
Raman Spectroscopy: Based on the Raman scattering effect. By measuring the frequency change of Raman scattered light, the vibration information of molecules can be obtained.
Hardware composition
FTIR: the hardware composition is more complex, usually including light source, interferometer, sample chamber, detector and other parts.
Raman spectrometer: It is mainly composed of light source, sample chamber, spectroscopic system, detector and other parts.
Applications
FTIR:
It is a fast and relatively inexpensive analytical technique that can be used to identify functional groups in molecules. With advances in technology and the discovery of universal attenuated total reflection techniques, the range of applications for FTIR has expanded dramatically, and analysis is often performed with little or no sample preparation.
Raman Spectroscopy:
Identifies any solid or liquid chemical in seconds, is insensitive to water, does not require removal of the sample from the container, and the sample is not analytically damaged or tampered with. Has a rechargeable battery, is fast and easy to sterilize, and the user interface is designed to be used while wearing protective gear.

What Is The Difference Between FTIR And ATR?
Principle
FTIR: FTIR is a technique for obtaining infrared spectra based on the Fourier transform of an interferogram.
ATR: ATR is an infrared spectroscopy technique based on the principle of attenuated total reflection.
Sample Preparation
FTIR: For solid samples, milling, mixing and tabletting with KBr is usually required, which is a time consuming and potentially destructive process.
ATR: The ATR technique eliminates the need for complex sample preparation by placing solid or liquid samples directly on the ATR crystal.
Areas of Application
FTIR:
In the field of chemical analysis to determine the structure of compounds, to analyze chemical reaction processes, to detect impurities, and more.
In materials science to study the composition, structure and properties of materials.
In the field of biomedicine to analyze the structure and function of biomolecules.
ATR
Microbial biochemistry and biotechnology field, food industry, protein research, characterization of insulating paper, origin identification of heavy building, in-situ measurement of solution concentration aspects, drug photodegradation research, amyloid research, analysis of silk materials.

What Is The Difference Between Kbr And ATR In FTIR Spectroscopy?
Sample Preparation:
KBR: Samples are usually mixed with KBr powder and pressed into tablets, a relatively tedious and time consuming process.
ATR: Samples can be assayed directly without the need for complicated sample preparation.
Assay suitability aspects:
KBR: More suitable for determining the proportion of components in a sample.
ATR: Excellent in rapid species identification.
Interference factor aspects:
KBR: The KBr technique can be problematic when the sample chemically reacts with the matrix in the KBr, or when absorption bands in the suspension medium interfere with the sample spectra.
ATR: Relatively less subject to interfering factors, but in practice may also be affected by the surface state of the sample, refractive index, etc.

Does FTIR Spectroscopy Destroy The Sample?
Benchtop FTIR is less destructive to the sample under test in most cases, but may affect the sample under test to a certain extent during sample collection and handling due to improper handling.

Does Concentration Affect FTIR Spectroscopy?
Changes in gas concentration can lead to changes in spectral characteristics, which can affect the column concentration values obtained by inversion. Also, when gases of different concentrations are mixed together, it will also affect the FTIR measurement results.
Changes in the concentration of liquids can affect the FTIR results for protein structure and other components.
Changes in the concentration of solids affect the FTIR results of their chemical structure.

What Causes Peak Shift In FTIR?
The nature of the material itself: different materials will show different peak characteristics in the FTIR spectrum. And the aging of the material will cause the FTIR peak to change.
Temperature: Temperature is one of the important factors affecting the change of FTIR peak.
Environmental factors: pollution level, humidity, air pressure.

What Is FTIR Spectroscopy?
FTIR Spectroscopy, also referred to as Fourier Transform Infrared Spectroscopy or FTIR Analysis, is a method of analysis employed for the identification of organic, polymeric, and inorganic substances. FTIR instruments capture frequencies all at once, leading to quicker data collection, better signal to noise ratios, and more precise wavenumber readings.

How Much Does An FTIR Spectroscopy Instrument Cost?
FTIR spectrometer price typically range from $15,000 to $145,000, depending on its resolution, sensitivity, automation or software, brand, model. For seeking a cost-effective option, you can ask your preferred FTIR manufacturer and supplier to provide a model and quotation of FTIR machine according to your requirements.

What Is FTIR Spectroscopy Used For?
These applications of FTIR spectrometer demonstrate its importance as a versatile and efficient analytical tool that provides fast, accurate chemical information to help industries improve product quality, optimize production processes, and ensure environmental safety.
Pharmaceutical industry:
For drug development, quality control and biomedical research. It can analyze the composition, structure and purity of drugs.
Drug composition analysis: FTIR machine is used to confirm the chemical structure of APIs and to ensure the purity and quality of chemicals used in the production process.
Quality control: monitoring the quality of drugs in the production process to ensure that the final product meets the preset chemical and physical standards.
Formulation development: Analyzing the interaction of ingredients in drug formulations to optimize drug formulations.
Minerals:
For mineral analysis to help identify mineral types and structures.
Petroleum:
Crude oil and fuel analysis: Analyzing the chemical composition of crude oil and petrochemical products to optimize processing. Analysis of petroleum products to detect organic functional groups and chemical composition.
Coal:
For coal analysis to study the content and structure of organic matter.
Environmental protection:
Pollutant Detection: Monitoring and analyzing pollutants in air, water and soil samples, such as industrial emissions and detection of hazardous chemicals.
For environmental monitoring to analyze pollutants in the atmosphere, water and soil.
Gem identification:
Detect the lattice structure and composition of gemstones and identify the authenticity of gemstones.
Criminal investigation identification:
Analyze the composition of unknown substances to help solve cases.
Conservation and Archaeology:
Material analysis of artifacts: Determine the material composition of ancient objects such as paintings, sculptures, and other artifacts to guide conservation and restoration work.
Food industry:
Ingredient verification: Determining the composition of foodstuffs, such as fats, proteins and other nutrients.
Adulteration Detection: Detects whether food products contain illegally added substances or are adulterated with low-cost substitutes.
Chemical industry:
Chemical analysis: to confirm the structure and purity of products of chemical reactions and raw materials.
Process control: Monitor intermediates and products in chemical processes to optimize productivity and product quality.
Polymer and plastic industry: material performance evaluation, analyze the chemical structure of plastics and polymers, predict and test their physical properties.
Research and development of new materials: develop new plastic formulations and composite materials to meet specific industrial needs.

What Is The Working Principle Of FTIR Spectroscopy?
FTIR spectrometry is an infrared spectroscopy analysis instrument based on the principle of interferometer and Fourier transform.
Interferometer part: FTIR testing generates two coherent infrared light beams with a small optical path difference through the Michelson interferometer. After passing through the sample, these two beams of light interfere with each other to form an interference pattern containing sample information.
Fourier transform part: The interference pattern is input into the computer, and the time domain function (interference pattern) is converted into a frequency domain function (infrared spectrum) through the Fourier transform algorithm. In this way, the infrared spectrum information of the sample can be obtained.

What Is The Structure Of FTIR Spectroscopy?
The basic structure of FTIR spectroscopy instrumenation consists of infrared light source, beam splitter, interferometer, cuvette, detector, computer data processing system and recording system.
1. Infrared light source: provide infrared radiation, commonly used tungsten lamps, tungsten iodine lamps, silicon carbon rods, high-pressure mercury lamps and thorium oxide lamps.
2. Beam splitter: Divide the incident beam into two parts, reflection and transmission, and then make it compound.
3. Interferometer: The interferogram of incident light is obtained using a Michelson interferometer.
4. Sample cell: Place the sample to be tested to ensure that the sample is under the irradiation of infrared light.
5. Detector: Convert the optical signal into an electrical signal. Commonly used detectors are titanium sulfate triglyceride (TGS), barium strontium niobate, mercury cadmium telluride, indium antimonide and so on.
6. Computerized data processing system: control the operation of the instrument, collect and process data.
7. Recording system: record and display the spectral map, in order to analyze and explain.

What Does FTIR Spectroscopy Use To Measure?
FTIR analysis, a widely used technique nowadays, is favored for its sensitivity, versatility, precision, and resilience. It is capable of analyzing solids, liquids, and gases, making it a highly utilized analytical method in the field of science.(Such as Acetone, Methanol, Nitromethane, Benzene, Cyclohexane, Ether, Carbonyl, Polystyrene, Acetic acid, Aspirin, Polypropylene, Polyethylene, Carboxylic acid, Ethyl acetate, Hexane, Amide, Benzoic acid, Methane, Cinnamaldehyde, Ester, Pvc, PP, Silicate FTIR)

What Cannot Be Detected By FTIR Spectroscopy ?
Certain Low Concentration Substances: ATR FTIR spectrometer test has limited detection sensitivity and may have difficulty detecting substances at very low concentrations.
Substances with similar structure: For substances with very similar structure, benchtop FTIR may be difficult to distinguish. In such cases, it may not be possible to accurately determine exactly which substance is involved by relying solely on FTIR testing.
Specific components in partially complex mixtures: In complex mixtures, certain substances may be interfered with by the signals of other components, making them difficult to detect.
Colorless, odorless substances with weak infrared absorption: Some colorless, odorless substances with weak absorption in the infrared spectral region may be difficult to detect by FTIR machine, such as simple cations and anions.
Certain Special Forms of Substances: FTIR spectrometer may be limited in the detection of certain special forms of substances, such as nanoscale particles or ultra-thin films. Metals and some minerals don't absorb light in the infrared range, so they can't be detected by FTIR analyzer. Diatomic or noble gases can not be analysis too.
Substances under extreme conditions such as high temperatures and pressures: Under extreme conditions such as high temperatures and pressures, the structure and properties of a substance may change, thus affecting the FTIR detection results.
Substances with strong fluorescence or phosphorescence: Fluorescence and phosphorescence produce strong light signals, which may mask the absorption signals of infrared light, thus affecting the FTIR detection results.

Can FTIR Spectroscopy Be Used For Qualitative Or Quantitative Analysis?
ATR FTIR spectrometer is well-known for its ability to do qualitative analysis, but it can also be used to do quantitative analysis. Researchers can create calibration curves by analyzing the connection between the strength of infrared absorption bands and the amount of a substance present, allowing for the accurate quantification of target compounds.

What Precautions Should Be Taken When Using FTIR Spectroscopy?
Before Use:
Proper Sample Preparation: Ensure that no impurities are introduced or contamination of the sample is caused during sample preparation.
During use:
Operator Safety: ATR FTIR instruments typically use an infrared light source, and operators should avoid direct exposure to infrared light to avoid eye and skin damage. Appropriate protective eyewear and gloves should be worn when operating the instrument. Not using explosive or flammable samples, not placing anything on top of the electronics cover
Instrument Safety: Ensure that the power connection to the FTIR spectrophotometer is stable to avoid electrical accidents. Be careful when moving or handling the instrument to avoid collision and damage.
Instrument parameter settings: Select these parameters according to the different analytical purposes and sample characteristics.
Baseline correction: In FTIR analyzer, baseline drift may affect the accurate measurement of peak positions and intensities. Choose an appropriate baseline correction method to ensure that the corrected spectrum can accurately reflect the characteristics of the sample.
Peak identification and attribution: Accurate identification and attribution of absorption peaks in an FTIR spectrum is critical to analyzing a sample.
Use of data analysis software: Choosing the appropriate data analysis software can improve the efficiency and accuracy of data processing.
After use:
Instrument maintenance and calibration: Maintain and calibrate the FTIR spectrometry regularly to ensure stable performance and accurate measurement results.
Application Specific Precautions
Cultural relics protection field: in the protection of cultural relics, FTIR technology can be used to analyze the material composition of cultural relics, degradation and so on. However, attention needs to be paid to the protection of cultural relics when using it to avoid damage to the relics.
Medical field: In the medical field, it can be used for disease diagnosis, drug analysis and so on. However, the complexity and diversity of the samples, as well as medical ethics and other issues need to be considered when using it.
Environmental monitoring field: In environmental monitoring, it can be used to detect atmospheric pollutants, water quality and so on. However, attention should be paid to the influence of environmental factors on the measurement results, such as temperature and humidity.
Material Science: In material science, it can be used to analyze the structure and composition of materials. However, when using it, it is necessary to pay attention to the influence of material properties and preparation methods on the measurement results.
Automotive engineering: In automotive engineering, it can be used to analyze the composition of engine exhaust gases. However, attention needs to be paid to the measurement conditions and data processing methods when using it to ensure the accuracy of the results.

How Does FTIR Spectrometer Work/Operate?
Let's take Cialan's FTIR-850 as an example.
1. Press the power switch to turn on the power supply, the instrument needs a self-test process after powering up, about 10 seconds. Wait for at least 15 minutes after the instrument is powered up, and wait for the light source energy output to be fully stabilized before measurement.
2. Turn on the computer and run the operation software. Check whether the communication between the computer and the instrument is normal.
3. Click the “acquisition” menu under the “acquisition settings”, select “Diagnostics” to observe whether the normal, the normal selection of the “optical table “, in the “optical table pane” to observe the energy value, to ensure that the energy value in the acceptable range, such as not in this range, it is necessary to click the “Diagnostics” in the “Collimation If not, you need to click the “Collimation” button in “Diagnostics” to perform the collimation operation.
4. Potassium bromide tablet method: take about 0.3mg of the test article, put it in the agate mortar, add dry potassium bromide, fully grind and mix it, move it to the press mold to make the distribution uniform, place the press mold horizontally on the seat of the tablet press, pressurize it to 10t/cm2, keep it for 3 minutes, take out the test tablet, and check it visually that it should be uniform, with a smooth surface, and with good transmittance of light.
5. Collect background: Click “Collect” menu under the “Collect Background”, appear to collect background prompt box, take out the sample from the sample chamber, insert the blank into it (or nothing), click “OK! Click “OK” to start collecting background.
6. Collect samples: Click the “Collection” menu under the “Collect Samples”, the collection of samples prompt box appears, take the blank out of the sample chamber, insert the sample. Click “OK” to start collecting samples. After the collection, the computer will automatically calculate and display the infrared spectrum of the sample on the spectrum window.
7. Data processing: After the acquisition is completed, the user can carry out the corresponding data processing on the spectrogram, such as labeling peaks, multiplying the spectrogram, smoothing, library search, etc.
8. Press the switch of the main power of the instrument to turn off the power of the main unit.
9. Remove the samples from the sample compartment, make sure the sample compartment is clean, and place bagged desiccant into the sample compartment.
10. Clean the spectrometer and molds; turn off the computer.
11. Make a record of instrument use.

How To Evaluate FTIR Results?
To analyze the results of FTIR spectra, the following steps can be followed:
1. Observe the overall characteristics of the spectrogram:
Examine the horizontal and vertical axes of the spectrogram to understand the relationship between wave number or wavelength and absorbance. Wave number is usually measured in centimeters-1 and absorbance is the degree to which light is absorbed by a substance.
Identify absorption peaks in a spectrogram that indicate the frequency at which a substance absorbs infrared light. The location and intensity of the absorption peaks can provide information about the functional groups of the substance.
2. Identify characteristic absorption peaks:
Each functional group has specific infrared absorption peaks, for example:
Hydroxyl (-OH) usually has an absorption peak between 3200-3600 cm-1.
Carbonyl (C=O) usually has an absorption peak between 1600-1800 cm-1.
Methyl (-CH3) usually has an absorption peak between 2800-3000 cm-1.
Identify functional groups that may be present in the sample by comparing known standard spectra or using specialized software.
3. Analyze the details of the spectrogram:
Check whether the baseline of the spectrogram is smooth, if there is drift or trailing, appropriate processing (e.g. automatic baseline correction and smoothing) is required.
Pay attention to peak shapes and symmetries in the spectrogram; these features can provide information about the structure of the molecule.
If required, the spectrogram can be further analyzed quantitatively, e.g., by calculating the peak areas to determine the content of the substance.
4. Combination with other analytical techniques:
FTIR spectrograms are often used in combination with other analytical techniques to obtain more comprehensive information about a substance.

How To Read FTIR Spectra?
1. Identification of functional groups: Based on the position and shape of the absorption peaks, the presence of functional groups or chemical bonds in the sample can be initially identified.
2. Comparison of samples: The spectra of the samples to be measured are compared with known standards or literature data to confirm the chemical composition and structure of the samples.
3. Quantitative analysis: Through the comparison of peak intensity and integral area, quantitative analysis can be carried out, such as determining the content of a functional group or compound in the sample.
4. Structural inference: According to the position and intensity of the absorption peaks, the molecular structure, conformation and chemical environment of the sample can be inferred.

How Do You Identify FTIR Peaks?
1. Position of peaks (wave number): The position at which each peak appears (expressed in wave number, i.e., cycles per centimeter, cm -1,) tells us which type of chemical bond or functional group is absorbing. The wave number is an indicator of the energy state of the chemical bond, and different types of chemical bonds absorb infrared light in different wave number regions.
2. The shape and width of the peaks:
Sharp peaks: usually indicate the presence of purity in the sample
Sharp peaks: usually indicate the presence of compounds of higher purity in the sample, because the absorption peaks of specific chemical bonds of a single compound are usually sharp and well-defined. 4.
Wide peaks: may indicate that the compounds contained in the sample are more complex or mixtures, as multiple compounds are present.
Wide peaks: may indicate that the sample contains complex compounds or mixtures of compounds, as the overlapping absorption of multiple compounds can lead to broad peaks.
3. Intensity of peaks:
Strong peaks: If a peak is very strong, this usually indicates that the
If a peak is strong, this usually indicates a high concentration of the chemical bond or functional group.
Weak peaks: Conversely, a weak peak indicates a lower concentration of the chemical bond or functional group.
4. Number of peaks: The number of peaks can reflect the complexity of the sample. Multiple peaks may indicate that the sample contains multiple bonds or functional groups.

How To Calculate Frequency From FTIR Graph?
To calculate the frequency, start by acquiring the FTIR spectrum of your sample. Make sure the representation is precise by carrying out any required baseline adjustments. Recognize the significant peaks that correspond to functional groups or chemical bonds present in the sample. Begin by concentrating on the high-frequency portion of the spectrum, as this is important for identifying functional groups.
Once you have found the highest points, establish the area to focus on by observing the span of wavenumbers. This stage is crucial for separating particular vibrations for additional examination or evaluation. Utilize numerical integration methods to compute the extent beneath each line within the specified range. Adding up these extents will yield the overall area beneath the line, which corresponds to the sample's vibrational energy.
Keep in mind that the maximum resolution of your FTIR spectrometer, which is influenced by the movable mirror's range of motion, can impact the accuracy of your frequency measurements. Consider this variable to optimize resolution in

What Are The Advantages Of FTIR Spectroscopy?
High sensitivity: FTIR spectrometer boasts remarkable sensitivity, enabling the identification of minute quantities of substances within a sample.
Enhanced Frequency Accuracy: This technique offers superior frequency resolution and reliability, leading to more precise results.
Wide applications: FTIR spectroscopy is adaptable to a diverse range of samples, including solids, liquids, film and gases.
Non-destructive of Samples: As a that samples remain intact and unaltered throughout the analysis process, ideal for analyzing delicate.
Analysis times are remarkably short, typically spanning from a few seconds to a few minutes, facilitating rapid decision-making.
Quantitative analysis: FTIR spectroscopy allows for the precise quantification of compound concentrations within a sample, providing actionable insights.
Scan faster: yielding a significantly improved signal-to-noise ratio and higher-quality spectral data.
Spectral Storage and Processing: FTIR spectroscopy facilitates the storage and processing of spectral data, streamlining data management and analysis.

What Are The Disadvantages/Limitations Of FTIR Analysis?
ATR FTIR spectrometer is limited to identifying functional groups rather than individual molecules in a sample, making it unsuitable for determining the complete chemical structure of a compound.
Complex samples, such as mixtures or those with overlapping spectra, pose challenges in accurately identifying components.
Additionally, the sensitivity of FTIR to water means that samples must be thoroughly dried before analysis.
The technique also requires the preparation of a thin sample film, which can be time-consuming and necessitates expertise. Spectral interference from other molecules or impurities can lead to inaccurate measurements, requiring the material being tested to be transparent in the relevant spectral region.
The molecule under analysis must also exhibit activity in the infrared range.

What Is The Difference Between FTIR And IR?
Principle:
IR: The structure and composition of a substance is analyzed by using its absorption properties of infrared light at different wavelengths. By measuring the intensity of the sample's absorption of infrared light at different wavelengths, an infrared absorption spectrum can be obtained.
FTIR: Based on the Fourier Transform principle, the interferogram is converted into an infrared spectrum. an FTIR instrument usually consists of an infrared light source, an interferometer, a sample chamber, and a detector. an FTIR has higher resolution and faster scanning speeds, and can measure the entire infrared spectral range at the same time.
Resolution:
FTIR has a higher resolution: FTIR can achieve very high resolutions, typically 0.1 - 0.5 cm-¹ or even higher.
This enables FTIR to resolve very fine spectral features, which is useful for analyzing complex molecular structures and mixtures.
The resolution of IR is relatively low: conventional IR instruments typically have a resolution of a few centimeters-¹ or so. While this may be sufficient for simple analytical tasks, FTIR is more advantageous when high resolution is required.
Scanning speed:
FTIR scans quickly: FTIR utilizes the Fourier Transform technique to scan the entire infrared spectral range in a very short time. This makes FTIR ideal for rapid analysis and real-time monitoring.
Slow IR scanning speed: Conventional IR instruments usually scan for different wavelengths of infrared light point by point, which makes the scanning speed slow. This limits the use of IR in applications that require rapid analysis.
Areas of application:
Common application areas: IR and FTIR have a wide range of applications in several fields, including chemistry, materials science, biomedical, and environmental science.
Unique applications of Benchtop FTIR in certain fields: FTIR machine has unique applications in specific fields due to its higher resolution and faster scanning speed. In cell biology, FTIR can be used to analyze the chemical composition and structure of cells, to detect cellular lesions, and so on.

What Is The Difference Between NIR And FTIR?
Principle
NIR: Near-infrared spectroscopy is the multiplicative and combinatorial frequency absorption spectra of molecular vibration, mainly reflecting the multiplicative and combinatorial frequency vibration information of hydrogen-containing groups (e.g. C-H, O-H, N-H, etc.). Near-infrared light has low energy, and the interaction with molecules is mainly generated by the change of dipole moment caused by molecular vibration. Near-infrared spectroscopy is based on the absorption and scattering of near-infrared light by the material under test, and the spectral response of the sample in the near-infrared region is measured to obtain information on the chemical composition and physical properties of the sample.
FTIR: Fourier Transform Infrared Spectroscopy is based on the absorption properties of molecules to infrared light. When a sample is irradiated with infrared light, the chemical bonds in the molecules will absorb infrared light of a specific wavelength, causing a jump in the vibrational energy levels of the molecules. By measuring the degree of absorption of the sample to different wavelengths of infrared light, the infrared absorption spectrum of the sample can be obtained.FTIR technology utilizes the Fourier transform to convert the interferogram into a spectrogram, which has the advantages of high resolution, high sensitivity and fast measurement.
Technical characteristics
NIR:
Rapid analysis: measurements are fast and a measurement can usually be made in a few seconds, making it suitable for on-line testing and real-time monitoring.
Non-destructive: non-destructive to the sample, allowing non-destructive testing, suitable for precious samples or samples that need to be tested repeatedly.
Simple operation: the instrument is relatively simple to operate, without the need for complex sample preparation process, usually only need to carry out simple processing or direct measurement of the sample.
Multi-parameter simultaneous determination: Multiple chemical compositions can be determined simultaneously, providing rich sample information.
FTIR:
High resolution: with high resolution, it can distinguish subtle spectral differences, which is advantageous for the analysis of complex samples.
Wide applicability: Can analyze various types of samples, including solids, liquids and gases, suitable for applications in different fields.
Accurate structural analysis: able to provide molecular structure information, which plays an important role in the identification and structure analysis of unknown substances.
Strong quantitative analysis capability: accurate quantitative analysis is possible through the establishment of standard curves.

What Is The Difference Between FTIR Spectroscopy And Raman Spectroscopy?
Spectroscopic Principle Aspects
FTIR Fourier Transform Infrared Spectrometer: The vibrational information of molecules is obtained by measuring their absorption of infrared light. Different chemical bonds have specific vibrational frequencies, so that functional groups in a molecule can be identified by infrared spectroscopy.
Raman Spectroscopy: Based on the Raman scattering effect. By measuring the frequency change of Raman scattered light, the vibration information of molecules can be obtained.
Hardware composition
FTIR: the hardware composition is more complex, usually including light source, interferometer, sample chamber, detector and other parts.
Raman spectrometer: It is mainly composed of light source, sample chamber, spectroscopic system, detector and other parts.
Applications
FTIR:
It is a fast and relatively inexpensive analytical technique that can be used to identify functional groups in molecules. With advances in technology and the discovery of universal attenuated total reflection techniques, the range of applications for FTIR has expanded dramatically, and analysis is often performed with little or no sample preparation.
Raman Spectroscopy:
Identifies any solid or liquid chemical in seconds, is insensitive to water, does not require removal of the sample from the container, and the sample is not analytically damaged or tampered with. Has a rechargeable battery, is fast and easy to sterilize, and the user interface is designed to be used while wearing protective gear.

What Is The Difference Between FTIR And ATR?
Principle
FTIR: FTIR is a technique for obtaining infrared spectra based on the Fourier transform of an interferogram.
ATR: ATR is an infrared spectroscopy technique based on the principle of attenuated total reflection.
Sample Preparation
FTIR: For solid samples, milling, mixing and tabletting with KBr is usually required, which is a time consuming and potentially destructive process.
ATR: The ATR technique eliminates the need for complex sample preparation by placing solid or liquid samples directly on the ATR crystal.
Areas of Application
FTIR:
In the field of chemical analysis to determine the structure of compounds, to analyze chemical reaction processes, to detect impurities, and more.
In materials science to study the composition, structure and properties of materials.
In the field of biomedicine to analyze the structure and function of biomolecules.
ATR
Microbial biochemistry and biotechnology field, food industry, protein research, characterization of insulating paper, origin identification of heavy building, in-situ measurement of solution concentration aspects, drug photodegradation research, amyloid research, analysis of silk materials.

What Is The Difference Between Kbr And ATR In FTIR Spectroscopy?
Sample Preparation:
KBR: Samples are usually mixed with KBr powder and pressed into tablets, a relatively tedious and time consuming process.
ATR: Samples can be assayed directly without the need for complicated sample preparation.
Assay suitability aspects:
KBR: More suitable for determining the proportion of components in a sample.
ATR: Excellent in rapid species identification.
Interference factor aspects:
KBR: The KBr technique can be problematic when the sample chemically reacts with the matrix in the KBr, or when absorption bands in the suspension medium interfere with the sample spectra.
ATR: Relatively less subject to interfering factors, but in practice may also be affected by the surface state of the sample, refractive index, etc.

Does FTIR Spectroscopy Destroy The Sample?
Benchtop FTIR is less destructive to the sample under test in most cases, but may affect the sample under test to a certain extent during sample collection and handling due to improper handling.

Does Concentration Affect FTIR Spectroscopy?
Changes in gas concentration can lead to changes in spectral characteristics, which can affect the column concentration values obtained by inversion. Also, when gases of different concentrations are mixed together, it will also affect the FTIR measurement results.
Changes in the concentration of liquids can affect the FTIR results for protein structure and other components.
Changes in the concentration of solids affect the FTIR results of their chemical structure.

What Causes Peak Shift In FTIR?
The nature of the material itself: different materials will show different peak characteristics in the FTIR spectrum. And the aging of the material will cause the FTIR peak to change.
Temperature: Temperature is one of the important factors affecting the change of FTIR peak.
Environmental factors: pollution level, humidity, air pressure.